DEDIATEST 2019-nCoV IgG/IgM - made in Germany
Rapid lateral flow test for the qualitative detection of IgG and / or IgM antibodies for 2019 nCoV ( novel corona Virus ) in whole blood, serum and plasma.
Summary
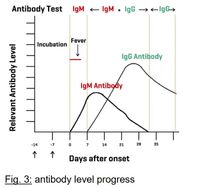
Early January 2020, a novel coronavirus (2019-nCoV) was identified as the infectious agent causing an outbreak of viral pneumonia in Wuhan, China, where the first cases had their symptom onset in December 2019. Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases. Six coronavirus species are known to cause human disease. Four viruses —229E, OC43, NL63, and HKU1 — are prevalent and typically cause common cold symptoms in immunocompetent individuals. The two other strains — severe acute respiratory syndrome coronavirus (SARS-COV) and Middle East respiratory syndrome coronavirus (MERS-COV) — are zoonotic in origin and have been linked to sometimes fatal illness. Coronaviruses are zoonotic, meaning they are transmitted between animals and people. Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death. Standard recommendations to prevent infection spread include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs. Avoid close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing.
Principle
The DEDIATEST 2019-nCoV IgG/IgM rapid test is a qualitative membrane-based immunoassay for the detection of IgG and IgM antibodies to 2019-nCoV in whole blood, serum or plasma specimen. This test consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in IgG test line region. During testing, the specimen reacts with 2019-nCoV antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti-human IgG in IgG test line region, if the specimen contains IgG antibodies to 2019-nCoV. A colored line will appear in IgG test line region as a result of this. Similarly, anti-human IgM is coated in IgM test line region and if specimen contains IgM antibodies to 2019-nCoV, the conjugate-specimen complex reacts with anti-human IgM. A colored line appears in IgM test line region as a result. Therefore, if the specimen contains 2019-nCoV IgG antibodies, a colored line will appear in IgG test line region. If the specimen contains 2019-nCoV IgM antibodies, a colored line will appear in IgM test line region. If the specimen does not contain 2019-nCoV antibodies, no colored line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
Equipment required but not provided
Timer, for measuring the time between application of the sample and interpretation of the results (10 to 20 min).
Testing procedure
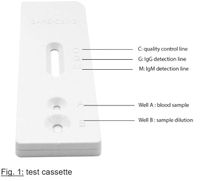
Add 20ul of whole blood sample or 10ul of serum or plasma sample to well A. Hold the dropper filled with buffer solution vertically above well B of the test cassette and add two drops (80μl) of buffer solution to well B. Wait until each drop is absorbed, before adding additional drops. Start the timer. Wait 10 minutes from addition of the buffer and then read the results. Warning: Do not interpret the results after more than 20 minutes, to avoid misinterpretation.
Interpretation of results
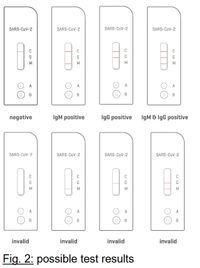
Positive (+): There appear red/purple line in both quality control area and either area M or G. Negative (-): There is only one red/purple line in the quality control area (C), and no line visible in either test area M and test area G. Invalid: There is no red/purple line in the quality control area (C), indicating incorrect operating procedures or the testing strip has already deteriorated. Under this conditions, read the instruction for use again carefully, and then use new test cassettes to test again. If the problem still exists, stop using this lot number immediately and contact your local suppliers.
Quality control
A process control is included in the test. A colored line appearing in the control area (C) is the internal process control. It confirms sufficient sample volume and correct technique.